Introduction
CenTRE offer services for administrative, regulatory and clinical aspects of the conduct of clinical trials. This will help our clinicians to ensure that clinical trials are performed to the best standards of clinical research, maximizing the opportunity for patients to avail themselves of the most modern and innovative treatment approaches. Clinicians will also have the opportunities to perform linkages and networking with healthcare professionals across the world.
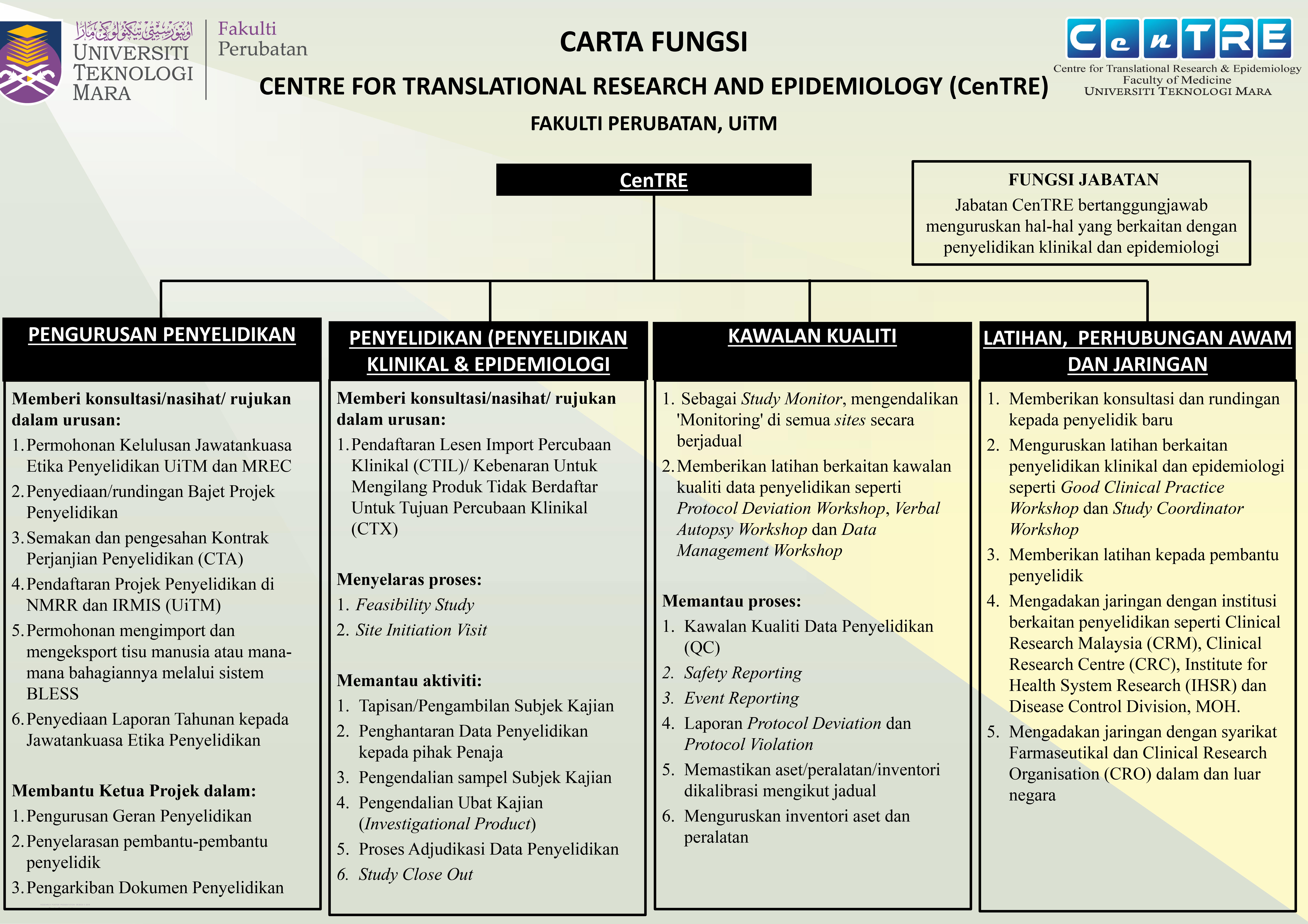
Services
| Research Administration | Advice Project Leader in applications for UiTM EC and MREC approvals |
|---|---|
| Advice Project Leader in Research Project Budget Preparation/Negotation | |
| Assist Project Leader in reviewing and endorsement of Clinical Trial Agreement | |
| Advice Project Leader in research project registration in NMRR and IRES (UiTM) | |
| Advice Project Leader in applications for importing and exporting human tissues or any part thereof through the BLESS system | |
| Assist Project Leader in managing Research Grant | |
| Assist Project Leader in managing Research Assistants | |
| Advice Project Leader in completion and submission of the Annual Research Report to UiTM EC/MREC | |
| Assist Project Leader in archiving of Research Documents | |
| Research Activities | Clinical Trial
|
Epidemiology
|
|
| Trainings, Public Relations and linkages | Coordinate trainings related to clinical and epidemiological research such as Good Clinical Practise Workshop and Study Coordinator Workshop |
| Provide necessary consultation to new researchers | |
| Provides training to new research assistants | |
| Establish networks with research-related institutions such as Clinical Research Malaysia (CRM), Clinical Research Center(CRC), Institute for Health System Research(HSR) and Disease Control Division, MOH | |
| Establish linkages with Pharmaceutical and Clinical Research Organisation(CRO) companies locally and abroad | |
| Quality Control | Provides training on data quality control such as Protocol Deviation Workshop, Verbal Autopsy Workshop and Data Management Workshop |
| Perform 'Monitoring' on all sites for IIR on a regular basis | |
| Monitor Data Quality Control (QC) | |
| Monitor Safety Reporting(Adverse Event and Serious Adverse Event) | |
| Monitor Event Reporting | |
| Monitor PRotocol Deviation and Protocol Violation report | |
| Ensure assets/qequipment/inventory are calibrated on schedule | |
| Manage inventory of assets and equipment |

