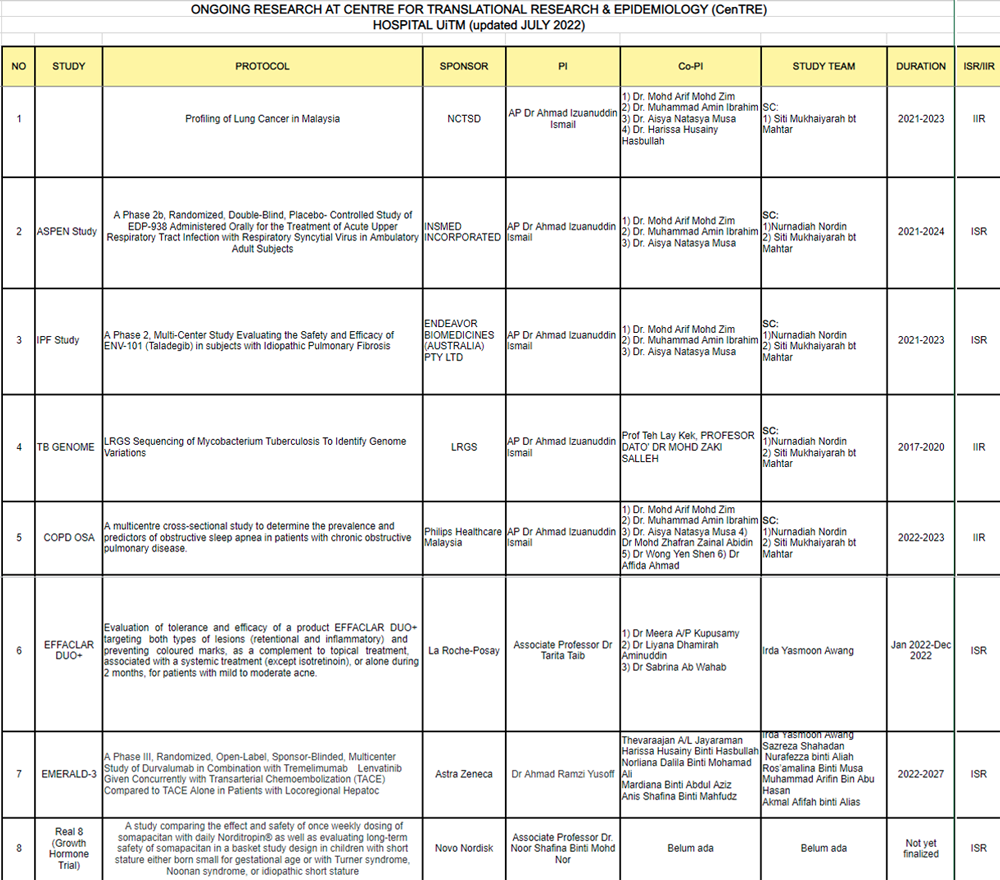
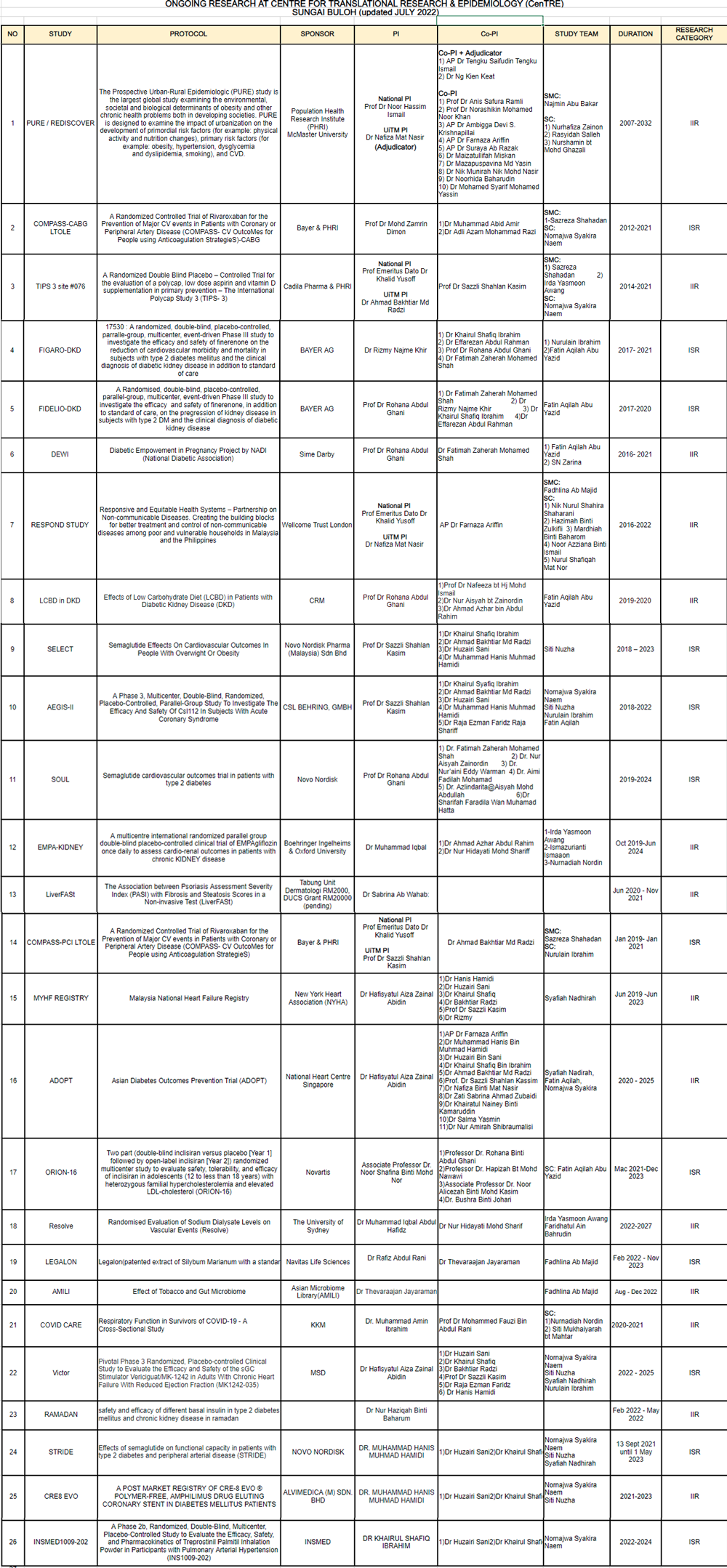
Research Grant
NO. |
STUDY |
PROTOCOL |
SPONSOR |
PI |
CO-PI |
TEAM STUDY |
DURATION |
STUDY LINK |
|
|
OnTARGET / TRANSCEND TRIALC6:C57 |
ONgoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial A Large, Simple Randomized Trial of an Angiotensin II Receptor Antagonist (Telmisartan) and an ACE-Inhibitor (Ramipril) in Patients at High Risk for CV Events. |
PHRI & Mc Master University, Canada |
Prof Dato’ Dr Khalid Yusoff |
|
|
2004-2008 |
https://clinicaltrials.gov/ct2/show/NCT00153101 |
|
|
SMARTER |
Symbicort Maintenance and Reliver Therapy, Experience in Real Life Setting in Malaysia |
Astra Zeneca |
AP Dr Tengku Saifudin Tengku Ismail |
|
|
2007-2009 |
https://clinicaltrials.gov/ct2/show/NCT00793624 |
|
|
RESPIMART STUDY |
A Randomized, Double-blind, Placebo Controlled, Parallel Group Study to Assess Long Term (1year) Efficacy and Safety of Tiotropium Inhalation Solution 5µg (2 puff of 2.5 µg) Delivered by the Respimart Inhaller in Patients in Chronic Obstructive Pulmonary Disease. |
Boehringer Ingelheim |
AP Dr Tengku Saifudin Tengku Ismail |
|
|
2007-2009 |
|
|
|
EPOCH Study |
Environmental Profile of a Community's Health (EPOCH): An Instrument to Measure Environmental Determinants of Cardiovascular Health in Five Countries. |
PHRI & Mc Master University, Canada |
Prof Dato’ Dr Khalid Yusoff |
|
1) Mohd Yazrie Yacob
|
2009 -2011 |
https://www.clinicaltrials.gov/ct2/show/NCT03225586 |
|
|
COPD |
A randomized, double-blind, double-dummy, placebo-controlled, parallel group study to assess the efficacy and safety of 48 weeks of once daily treatment of orally inhaled BI 1744 CL (5µg [2 actuations of 2.5µg] and 10µg [2 actuations of 5µg]) delivered by Repimat Inhaler and 48 weeks of twice daily Foradil (12µg) delivered by the Aerolizer Inhaler in patients with Chronic Obstructive Pulmonary Disease |
Boehringer Ingelheim |
AP Dr Tengku Saifudin Tengku Ismail |
Dr Wan Haniza |
|
2009-2011 |
https://clinicaltrials.gov/ct2/show/NCT00793624 |
|
|
ADOPT |
A Phase 3 Randomized, Double-blind, Parallel-group, Multi-center Study of The Safety and Efficacy of Apixaban for Prophylaxis of Venous Thromboembolism in Acutely Ill Medical Subjects During and Following Hospitalization (EC Reference number: NMRR-08-1034-2189)-Apixaban Dosing to Optimize Protection from Thrombosis |
Bristol Mayer Squibb |
Prof Dato’ Dr Khalid Yusoff |
Dr. Effarezan Abdul Rahman |
|
2009-2012 |
https://clinicaltrials.gov/ct2/show/NCT00457002 |
|
|
RIVAROX – ACS3001@ ATLAS II |
A Randomized, Double – blind, Event – Driven Multicenter Study to Evaluate the Efficacy and Safety of Rivaroxaban in Subject With a Recent Acute Coronary Syndrome. |
Johnson & Johnson Pharmaceutical Research & Development |
Prof Dato’ Dr Khalid Yusoff |
Dr. Effarezan Abdul Rahman |
|
2010 – 2011 |
https://clinicaltrials.gov/ct2/show/NCT00809965 |
|
|
ARISTOTLE |
A Phase 3, Active (Warfarin) Controlled, Randomized, Double-Blind, Parallel Arm Study to Evaluate Efficacy and Safety of Apixaban in Preventing Stroke and Systemic Embolism in Subjects with Nonvalvular Atrial Fibrillation |
Bristol Mayer Squibb |
Prof Dato’ Dr Khalid Yusoff |
1)Dr. Mohd Kamal Mohd Arshad |
|
2007-2012 |
https://clinicaltrials.gov/ct2/show/NCT00412984 |
|
|
SAKURA |
A Comparison of Symbicort Maintenance and Reliver Therapy (Symbicort Turbuhaler 160/4.5 µg, one inhalation bid plus as needed) and Symbicort Turbuhaler 160/4.5µg, one inhalation bid plus terbutaline Turbuhaler 0.4mg/inhalation as needed, for treatment of asthma - a 12 month, randomized, double-blind, parallel group, active - controlled, multinational phase III study in asthmatic patients aged 16 years and above. |
Astra Zeneca |
Assoc Prof Dr Tengku Saifudin |
1)Dr Wan Haniza |
|
2009-2012 |
https://clinicaltrials.gov/ct2/show/NCT00839800 |
|
|
TIOSPIR |
A Randomized, Active-controlled, Double-blind, Double-dummy, Parallel Group Design, Multi-center Trial to Compare the Efficacy and Safety of 2.5 mg and 5μg Tiotropium Inhalation Solution Delivered by the Respimat Inhaler with Tiotropium Inhalation Capsules 18μg Delivered by the Handihaler |
Boehringer Ingelhem |
AP Dr Tengku Saifudin Tengku Ismail |
1)Dr Wan Haniza |
|
2010-2012 |
https://clinicaltrials.gov/ct2/show/NCT01126437 |
|
|
SYR322_402 @ EXAMINE TRIAL |
A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate Cardiovascular Outcomes Following Treatment with Alogliptin in Addition to Standard of Care in Subjects with Type 2 Diabetes and Acute Coronary Syndrome |
TAKEDA Global Research and Development |
Prof Dato’ Dr Khalid Yusoff |
Dr. Effarezan Abdul Rahman |
|
2010-2014 |
https://clinicaltrials.gov/ct2/show/NCT00968708 |
|
|
APOLLO |
A Randomized Controlled Trial of Aliskiren in the Prevention of Major Cardiovascular Events in Elderly People - Aliskiren Prevention Of Later Life Outcomes |
PHRI & Novartis |
Prof Dato’ Dr Khalid Yusoff |
1)Dr. Effarezan Abdul Rahman |
|
2011-2012 |
https://www.phri.ca/research/apollo |
|
|
ALEPREVENT |
Phase 3B Study to Evaluate the Potential of Aleglitazar to Reduce Cardiovascular Risk in Patients with Stable CVD and Glucose Abnormalities |
F. Hoffman-La Roche |
Prof Dato’ Dr Khalid Yusoff |
1)Assoc Prof Dr Sazzli Sahlan Kasim 2)Dr. Mohd Kamal Mohd Arshad |
|
2012-2013 |
https://clinicaltrials.gov/ct2/show/NCT01715818 |
|
|
INTERSTROKE |
Importance of Conventional and Emerging Risk Factors for Stroke in Different Regions of the World and in Different Ethnic Groups: A Case-control Study |
PHRI & Mc Master University, Canada |
Prof Dato’ Dr Khalid Yusoff |
1)Dr. Annamalai Chandramouli |
Sazreza Shahadan |
2009-2014 |
https://www.phri.ca/research/interstroke |
|
|
ADVANCE-ON |
A post-trial observational study extension to ADVANCE, a factorial randomized controlled trial of blood pressure lowering with a fixed low-dose perindopril-indapamide combination and intensive glucose control with a modified-release gliclazide (gliclazide MR)-based regimen for the prevention of vascular disease among high risk individuals with type 2 diabetes mellitus. |
The George Institute for Global Health, Sydney, Australia |
Prof Dato’ Dr Khalid Yusoff |
|
|
2011-2014 |
https://clinicaltrials.gov/ct2/show/NCT00949286 |
|
|
BREATH 3082 |
A 12-month, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of Reslizumab (3.0 mg/kg) in the reduction of clinical asthma exacerbations and change in lung function in patients (12-75 years of age) with eosinophilic asthma. |
Cephalon Inc |
AP Dr Tengku Saifudin Tengku Ismail |
1)Dr Wan Haniza |
|
2011-2014 |
https://clinicaltrials.gov/ct2/show/NCT01287039 |
|
|
TAK-875 or GRAND Study |
A Multicenter, randomized, Double Blind, Placebo Controlled, Study to Evaluate CV outcomes of TAK-875 50 mg in Addition to standard of Care in Subjects with T2DM and with CVD or Multiple Risk Factors for CV Events |
TAKEDA Global USA |
Prof Dato’ Dr Khalid Yusoff |
1)Assoc Prof Dr Sazzli Sahlan Kasim |
|
2013-2014 |
https://clinicaltrials.gov/ct2/show/NCT01609582 |
|
|
CQMF149F2202 |
A randomized, double-blind, 12-week treatment, parallelgroup study to evaluate the efficacy and safety of QMF149 (150 μg/160 μg o.d.) compared with salmeterol xinafoate/fluticasone propionate (50 μg/500 μg b.i.d.) in patients with chronic obstructive pulmonary disease. |
Novartis |
AP Dr Tengku Saifudin Tengku Ismail |
1)Dr Ahmad Izuanuddin |
|
2013-2014 |
https://clinicaltrials.gov/ct2/show/NCT01636076 |
|
|
HOPE-4 RAPID APPRAISAL |
Multi-Method Health System (Rapid Appraisal) |
PHRI |
Prof Dato Dr Khalid Yusoff |
Dr Ng Kien Keat |
SMC: |
2012-2014 |
|
|
|
HOPE-3 |
Heart Outcomes Prevention Evaluation-3 |
PHRI |
Prof Dato Dr Khalid Yusoff |
Dr. Effarezan Abdul Rahman |
SMC: |
2007-2015 |
https://www.phri.ca/research/hope-3 |
|
|
C-SCADE 8 |
A Phase III, multicentre, international, randomised, parallel group, double blind cardiovascular safety of BI 10773 (10mg and 25mg administered orally once daily) compared to usual care in Type2 diabetes mellitus patients with increased risk |
Boehringer Ingelhem |
AP Dr Rohana Abdul Ghani |
Dr Ahmad Izuanuddin Ismail |
Vickyneswary Muniandy |
2010-2015 |
https://clinicaltrials.gov/ct2/show/NCT01131676 |
|
|
INTER-CHF SG BULOH |
An international registry to study the current causes, treatment, barriers to care, and outcome of heart failure in Africa, Asia and South America |
PHRI |
National PI |
Dr.Johan Rizwal Ismail |
SMC: |
2012-2015 |
https://www.phri.ca/research/inter-chf |
|
|
INTER-CHF SELAYANG |
An international registry to study the current causes, treatment, barriers to care, and outcome of heart failure in Africa, Asia and South America |
PHRI |
Prof Dato Dr Khalid Yusoff |
1) Dr.Hafisyatul Aiza |
SMC: |
2012-2015 |
https://www.phri.ca/research/inter-chf |
|
|
AUSTRI SG BULOH |
A Safety and Efficacy Study of Inhaled Fluticasone Propionate/Salmeterol Combination versus Inhaled Fluticasone Propionate in the Treatment of Adolescent and Adult Subjects with Asthma. |
GlaxoSmithKline Research & Development Limited |
Dr Faisal Mohd Fadzli |
1)Assoc Prof Dr Tengku Saifudin Tengku Ismail |
SMC: |
2012-2015 |
https://www.clinicaltrials.gov/ct2/show/NCT01475721 |
|
|
AUSTRI SELAYANG |
A Safety and Efficacy Study of Inhaled Fluticasone Propionate/Salmeterol Combination versus Inhaled Fluticasone Propionate in the Treatment of Adolescent and Adult Subjects with Asthma. |
GlaxoSmithKline Research & Development Limited |
Dr Ahmad Izuanuddin Ismail |
1)Assoc Prof Dr Tengku Saifudin Tengku Ismail |
SMC: |
2012-2015 |
https://www.clinicaltrials.gov/ct2/show/NCT01475721 |
|
|
VESTRI |
A Safety and Efficacy Study of Inhaled Fluticasone Propionate/Salmeterol Combination versus Inhaled Fluticasone Propionate in the Treatment of Paediatric Subjects with Asthma. |
GlaxoSmithKline Research & Development Limited |
Dr Faisal Mohd Fadzli |
1)Assoc Prof Dr Tengku Saifudin Tengku Ismail |
Nurnadiah Nordin |
2012-2015 |
https://clinicaltrials.gov/ct2/show/NCT01462344 |
|
|
BREATH 3085 |
An Open-Label Extension Study to Evaluate the Long-Term Safety and Efficacy of Reslizumab (3.0 mg/kg) as Treatment for Patients With Eosinophilic Asthma Who Completed a Prior Cephalon-Sponsored Study in Eosinophilic Asthma |
Cephalon Inc |
AP Dr Tengku Saifudin Tengku Ismail |
1)Dr Ahmad Izuanuddin |
|
2012-2015 |
https://clinicaltrials.gov/ct2/show/NCT01290887 |
|
|
ROFLUMILAST |
A 52-week, Double-blind, Randomized, Placebo Controlled Parallel Group Study to Evaluate the Effect of Roflumilast 500µg on Exacerbation Rate in Subjects with Chronic Obstructive Pulmonary Disease (COPD) Treated with a Fixed Dose Combination of Long-Acting Beta Agonist and Inhaled Corticosteroid (LABA/ICS) |
Forest Research Institute, Inc |
Dr Ahmad Izuanuddin Ismail |
1)Assoc Prof Dr Tengku Saifudin Tengku Ismail |
1)Irda Yasmoon Awang |
2012-2015 |
https://www.mayo.edu/research/clinical-trials/cls-20112131 |
|
|
SUMMIT |
A Clinical Outcomes Study to compare the effect of Fluticasone Furoate/Vilanterol Inhalation Powder 100/25mcg with placebo on Survival in Subjects with moderate Chronic Obstructive Pulmonary Disease (COPD) and a history of or at increased risk for cardiovascular disease |
GlaxoSmithKline Research & Development Limited |
Dr Ahmad Izuanuddin Ismail |
1)Assoc Prof Dr Tengku Saifudin Tengku Ismail |
1)Irda Yasmoon Awang |
2014-2015 |
https://clinicaltrials.gov/ct2/show/NCT01313676 |
|
|
CNTO |
A Randomized, Placebo-controlled, Double-blind, Multi-center, Phase 2 Study to Assess the Efficacy and Safety of CNTO 6785 in Subjects With Moderate to Severe Chronic Obstructive Pulmonary Disease |
Janssen Research & development,LLC |
Dr Ahmad Izuanuddin Ismail |
1)Assoc Prof Dr Tengku Saifudin Tengku Ismail |
1)Nurnadiah Nordin |
2014-2015 |
https://clinicaltrials.gov/ct2/show/NCT01966549 |
|
|
SOLITAIRE |
A Randomized, Double-Blind, Multi-Center Study to Evaluate the Efficacy and Safety of Intravenous to Oral Solithromycin (CEM-101) Compared to Intravenous to Oral Moxifloxacin in the Treatment of Adult Patients with Community-Acquired Bacterial Pneumonia |
Chempra Pharmaceutical |
Dr Ahmad Izuanuddin Ismail |
1)Assoc Prof Dr Tengku Saifudin Tengku Ismail |
1)Nur Asimah Zainal Abidin |
2014-2015 |
https://www.clinicaltrials.gov/ct2/show/NCT01968733 |
|
|
CRLX030A2302 RELAX ASIA |
A Multi center, randomized, double-blind, placebo controlled phase III study to evaluate the efficacy, safety and tolerability of serelaxin when added to standard therapy in acute heart failure patients. |
Novartis |
AP Dr Sazzli Shahlan Kasim |
1)Dr.Johan Rizwal Ismail |
1) Siti Masnirah Alwi |
2013-2016 |
https://clinicaltrials.gov/ct2/show/NCT01870778 |
|
|
MASCOT Registry |
Multinational Abluminal Sirolimus Coated BiO-Engineered StenT –The MASCOT Post Marketing Registry |
Orbus-Neich Medical Inc. |
AP Dr Sazzli Shahlan Kasim |
1)Dr.Johan Rizwal Ismail |
Zureena Rahim |
2015 – 2016. |
https://clinicaltrials.gov/ct2/show/NCT02183454 |
|
|
QUALIFY |
Quality of adherence to guideline recommendations for life-saving treatment in heart failure: an national survey, "QUALIFY Registry” |
Novatech |
AP Dr Sazzli Shahlan Kasim |
1)Dr.Johan Rizwal Ismail |
Siti Masnirah Alwi |
2014-2017 |
|
|
|
CANVAS-R |
CaNagliflozin cardioVascular Assessment Study-Renal A Randomized, Multicenter, Double-Blind, Parallel, Placebo-Controlled Study of the Effects of Canagliflozin on Renal Endpoints in Adult Subjects with Type 2 Diabetes Mellitus |
Janssen Research & development,LLC |
Prof Dato Dr Khalid Yusoff |
Dr.Rizmy Najme Khir |
1)Sazreza Shahadan |
2014-2017 |
https://clinicaltrials.gov/ct2/show/NCT01989754 |
|
|
COROFLEX ISAR INTERNATIONAL REGISTRY |
Coroflex Isar International Registry is to assess the safety and efficacy of elective deploument of the Sirolimus-eluting Coroflex ISARTM Stent in the treatment of ‘real world’ de-novo and restenotic lesions after stand-alone angioplasty in coronary arteries |
B.Braun Medical Supplies Sdn Bhd |
AP Dr Sazzli Shahlan Kasim |
1)Dr.Johan Rizwal Ismail |
Siti Masnirah Alwi |
2015-2017 |
https://clinicaltrials.gov/ct2/show/NCT02629575 |
|
|
DPNP |
An Asian.Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled 14-Week Study of DS-5565 in patients withDiabetic Peripheral Neuropathic Pain followed by a 52-Week Open-Label Extension - (DPNP) |
Daiichi Sankyo |
AP Dr Rohana Abdul Ghani |
Dr.Annamalai Chandramouli |
|
2015 - 2017 |
https://clinicaltrials.gov/ct2/show/NCT02318706 |
|
|
RECOPD |
Risk Factor of Recurrent Exacerbation |
Malaysian Thoracic Society |
Dr Ahmad Izuanuddin Ismail |
1)Dr Mohd Arif Mohd Zim |
1) Nur Nadiah Nordin |
2012-2018 |
|
|
|
ODYSSEY (SG BULOH) |
Phase III study which is a randomized, double blind, placebo controlled, parallel group study to evaluate the effect of study drug on the occurrence of CV events in patients who have recently experience an ACS (EFC11570) Drug SAR236553 |
Sanofi Aventis R&D |
National PI |
1)Dr Johan Rizwal Ismail |
1) Nurhasanah Abdul Rahman |
2013-2018 |
https://clinicaltrials.gov/ct2/show/NCT01663402 |
|
|
ODYSSEY (SELAYANG) |
Phase III study which is a randomized, double blind, placebo controlled, parallel group study to evaluate the effect of study drug on the occurrence of CV events in patients who have recently experience an ACS (EFC11570) Drug SAR236553 |
Sanofi Aventis R&D |
National PI |
Dr Effarezan Abdul Rahman |
SMC: |
2013-2018 |
https://clinicaltrials.gov/ct2/show/NCT01663402 |
|
|
mySTEMI |
Malaysia ST-Elevation Myocardial Infarction (MySTEMI) Network Program |
National Heart Association of Malaysia |
Dr Mohd Kamal Mohd Arshad |
1) Assoc Prof Sazli Kasim |
1)Siti Masnirah Alwi 2)Nurhasanah Abdul Rahman |
2016-2018 |
https://www.clinicaltrials.gov/ct2/show/NCT02597920 |
|
|
Pradaxa |
Non-interventional study describing patients´ perception on anticoagulant treatment and treatment convenience when treated with Pradaxa® or Vitamin K Antagonist for Stroke Prophylaxis in Atrial Fibrillation. |
Boehringer Ingreihm |
Dr Zubin Othman Ibrahim |
1) Assoc Prof Sazli Kasim |
Siti Masnirah Alwi |
2016-2018 |
|
|
|
HAVE |
Hormonal Abnormalities Amongst HIV-Infected Individuals (HAVE) Horm (one-off study) |
Initiated Study (LESTARI) |
AP Dr Rohana Abdul Ghani |
Dr Rosdina |
Nur Atikah Bt Dolah |
2016-2018 |
|
|
|
EDIFIED |
The Effects of Dapagliflozin (SGLT-2 Inhibitor) on Endothelial Dysfunction in Type 2 Diabetes Mellitus |
Astra Zeneca (Malaysia) |
AP Dr Rohana Abdul Ghani |
Dr Nor Aisyah Zainordin |
Fatin Aqilah Abu Yazid |
2016-2018 |
|
|
|
METABOLOMIC |
Metabolomic Profiling in Type 2 Diabetes Mellitus Patients in UiTM Medical Centre |
Investigator Initiated Study (BESTARI) |
AP Dr Rohana Abdul Ghani |
Dr Fatimah Zaherah Mohamed Shah |
Fatin Aqilah Abu Yazid |
2016-2018 |
|
|
|
EMBRACE |
EMBRACE (Empagliflozin-Obtaining real health care data) |
Boehringer Ingelheim (Malaysia) Sdn Bhd |
AP Dr Rohana Abdul Ghani |
|
Nur Atikah Bt Dolah |
2017-2018 |
|
|
|
MASKID |
The Effectiveness of Mangosteen Skin Product (Spray8) on Diabetic Wound Healing |
Furley Bioextract Sdn Bhd & Platcom Venture |
Dr Wan Najwa Binti Wan Mohd Zohdi |
Dr Nor Faridah Binti Ahmad Roslan |
Nur Faridah Yusof |
2018 (terminated) |
|
|
|
HOPE-4 STUDY |
Heart Outcomes Prevention and Evaluation: HOPE-4 |
CIHR & Grand Challenges Canada as part of the Global Alliance for Chronic Disease |
National PI |
|
SMC: |
2015-2019 |
https://www.phri.ca/research/hope-4 |
|
|
COMPASS SG BULOH |
A Randomized Controlled Trial of Rivaroxaban for the Prevention of Major CV events in Patients with Coronary or Peripheral Artery Disease (COMPASS- CV OutcoMes for People using Anticoagulation StrategieS) |
Bayer & PHRI |
National PI |
1)Dr.Johan Rizwal Ismail |
SMC: |
2014-2019 |
https://www.phri.ca/research/compass |
|
|
COMPASS SELAYANG |
A Randomized Controlled Trial of Rivaroxaban for the Prevention of Major CV events in Patients with Coronary or Peripheral Artery Disease (COMPASS- CV OutcoMes for People using Anticoagulation StrategieS) |
Bayer & PHRI |
National PI |
1) Dr.Rizmy Najme Khir |
SMC: |
2014-2019 |
https://www.phri.ca/research/compass |
|
|
EXCSEL |
A randomized placebo controlled in clinical trial to evaluate cardiovascular outcome after treatment with Exenatide once weekly in patient with Type 2 diabetes mellitus. |
Astrazeneca |
Prof Dr Rohana Abdul Ghani |
|
Fatin Aqilah Abu Yazid |
2014-2019 |
|
|
|
HOPE 3 - Passive Follow-up |
Heart Outcome Prevention Evaluation 3 - Passive Follow-up |
PHRI |
National PI |
|
Nornajwa Syakira Naem |
2015-2019 |
https://www.phri.ca/research/hope-3-passive-follow-up |
|
|
VICTORIA |
A randomized Parallel-Group, Placebo-Controlled, Double-Blind, Event-driven, Multi-Center Pivotal Phase III Clinical Outcome Trial of Efficacy and Safety of the Oral sGC Stimulator Vericiguat in Subjects with Heart Failure with Reduced Ejection Fraction (HFrEF) - Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction |
Merck, Sharp & Dohme (M) Sdn Bhd |
Dr Hafisyatul Aiza Zainal Abidin |
1)Assoc Prof Sazzli Kasim |
1) Nurhasanah Abdul Rahman |
2016-2019 |
https://clinicaltrials.gov/ct2/show/NCT02861534 |
|
|
INFLUENZA |
1 year Hospital based surveillance for influenza cases in chronic obstructive pulmonary disease, asthmatic and community acquired pneumonia adult patients in Malaysia |
Sanofi Pasteur |
AP Dr Ahmad Izuanuddin Ismail |
1) Dr. Mohd Arif Mohd Zim |
SMC: |
2018-2019 |
|
|
|
CORRECT |
Phenotyping COPD patients using specific COPD Assessment Tool Scores |
UiTM Geran Lestari |
Dr. Aisya Natasya Musa |
Dr. Muhammad Amin Ibrahim |
1)Irda Yasmoon Awang |
Jul 2018-Dec 2019 |
|
|
|
CHRONIC COUGH (MK7264-030) |
A phase 3, randomized, double, placebo-controlled twelve months study to evaluate the efficacy and safety of MK-7264 in adult participants with chronic cough. |
MSD |
AP Dr Ahmad Izuanuddin Ismail |
1) Dr. Mohd Arif Mohd Zim |
1) Siti Mukhaiyarah bt Mahtar 2)Nurnadiah Nordin |
May 2019 |
https://clinicaltrials.gov/ct2/show/NCT03449147 |
|
|
CAIN |
A randomized, double-blind, placebo controlled, multicenter study of subcutaneous secukinumab, to demonstrate efficacy after twelve weeks of treatment and to assess safety, tolerability and long-term efficacy up to one year in subjects with moderate to severe chronic plaque-type psoriasis with or without psoriatic arthritis comorbidity |
Novartis |
Dr Tarita Taib |
1) Dr W. Syameen Amira |
Irda Yasmoon Awang |
Feb 2017-Jun 2019 |
https://clinicaltrials.gov/ct2/show/NCT0306660 |
|
|
CREDENCE |
A Randomized, Double-blind, Event-driven, Placebo-controlled, Multicenter study of effect of Canagligflozin on renal and cardiovascular outcomes in subject which Type 2 Diabetes Mellitus and diabetic nephropathy |
Janssen Research & development,LLC |
Assoc Prof Dr Rohana Ghani |
|
|
2015-2017 |
https://clinicaltrials.gov/ct2/show/NCT02065791 |
|
|
INFLUENZA |
1 year Hospital based surveillance for influenza cases in chronic obstructive pulmonary disease, asthmatic and community acquired pneumonia adult patients in Malaysia |
Sanofi Pasteur |
AP Dr Ahmad Izuanuddin Ismail |
1) Dr. Mohd Arif Mohd Zim |
SMC: SC: |
Jul 2018-Jan 2020 |
|
|
|
SABINA |
An observational, multi-country, cross sectional study describing SABA prescription and potential effects among asthma patients, using real-time electronic data collection methods in local health care settings |
AstraZeneca |
Dr Mohd Arif Mohd Zim |
1)AP Dr Ahmad Izuanuddin Ismail |
SC: |
Oct 2019-2021 |
https://astrazenecagrouptrials.pharmacm.com/ST/Submission/View?id=25825 |
|
|
Global Aware |
GLOBAL AWARE – Emerging Markets (EM) (Adults With Atopic Dermatitis Reporting on Their Experience in Emerging Markets) |
Sanofi Aventis |
Dr Tarita Taib |
1) Dr Meera A/P Kupusamy |
Irda Yasmoon Awang |
Dec 2019-Dec 2020 |